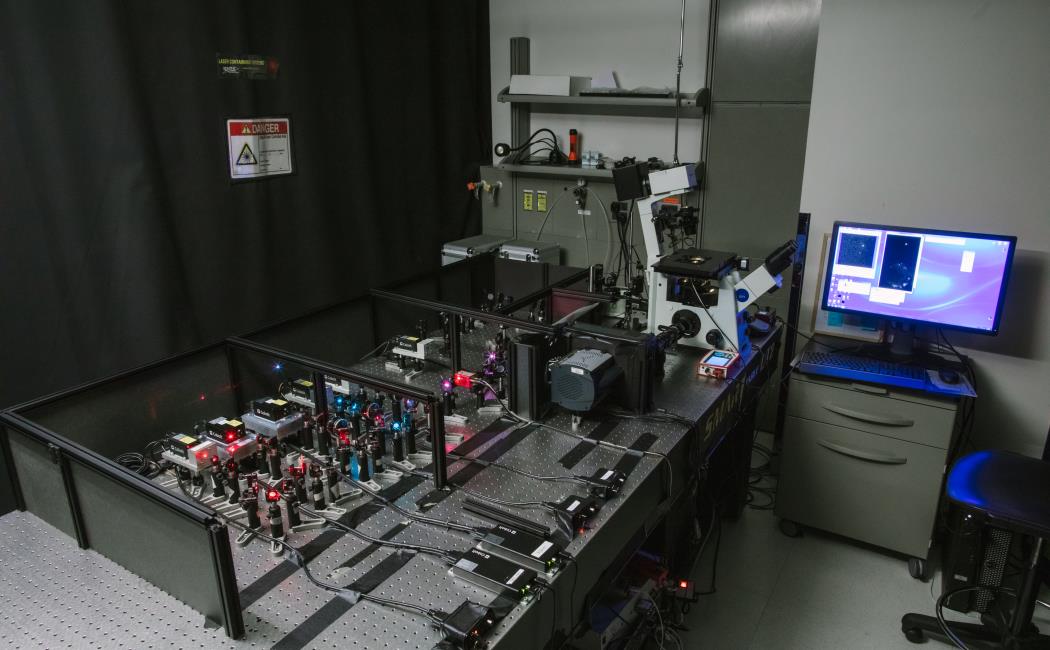
Custom-built wide-field epi-fluorescence microscope setup with five solid-state lasers with 638 nm, 561 nm, 532nm, 488 nm, and 405 nm (MLDTM, Cobolt). Acoustic-optical tunable filter (AOTF; AA Optoelectronic) is used to control the intensity of the individual laser lines for excitation. The collimated laser beams are introduced into the microscope through an achromatic focusing lens, providing widefield Köhler illumination of the sample. The fluorescence images are recorded using an inverted IX71 microscope (Olympus) with a different type of objective lens and illumination condition (TIRF or low angel). The fluorescence emitted from the sample is collected again by the same objective lens, and passed through OptoMask (Andor Technology) to enable capture up to three colors using the iXon Ultra 897 EM-CCD cameras (Andor Technology). The fluorescence light is separated from the laser excitation light using a dichroic mirror and an emission bandpass filter obtained from (Semrock). A cylindrical lens is placed in front of the iXon Ultra EMCCD camera to enable 3D astigmatism-based super-resolution fluorescence localization microscopy. The z-axis positions are controlled by a piezo nano-positioning stage (APZ-X00 Piezo Z-stage) (Andor). The image acquisition is done using either the SOLIS imaging software (Andor) or IQ2 from Andor. We inserted a commercial C-Focus lock system (Mad City Labs), to correct the microscopy focus drift over a long period of time by a high-resolution sensor system. The setup is equipped with variety of accuracies that enables us to measure live cell behavior in micro fluidics.